
Todd Bridges, RPh

Todd Bridges, RPh
Global President
Drug Safety Institute (DSI) is a wholly-owned regulatory subsidiary of Brand Institute. DSI provides a portfolio of industry-leading guidance pertaining to drug name safety (i.e., preventing medication errors), packaging, and labeling.

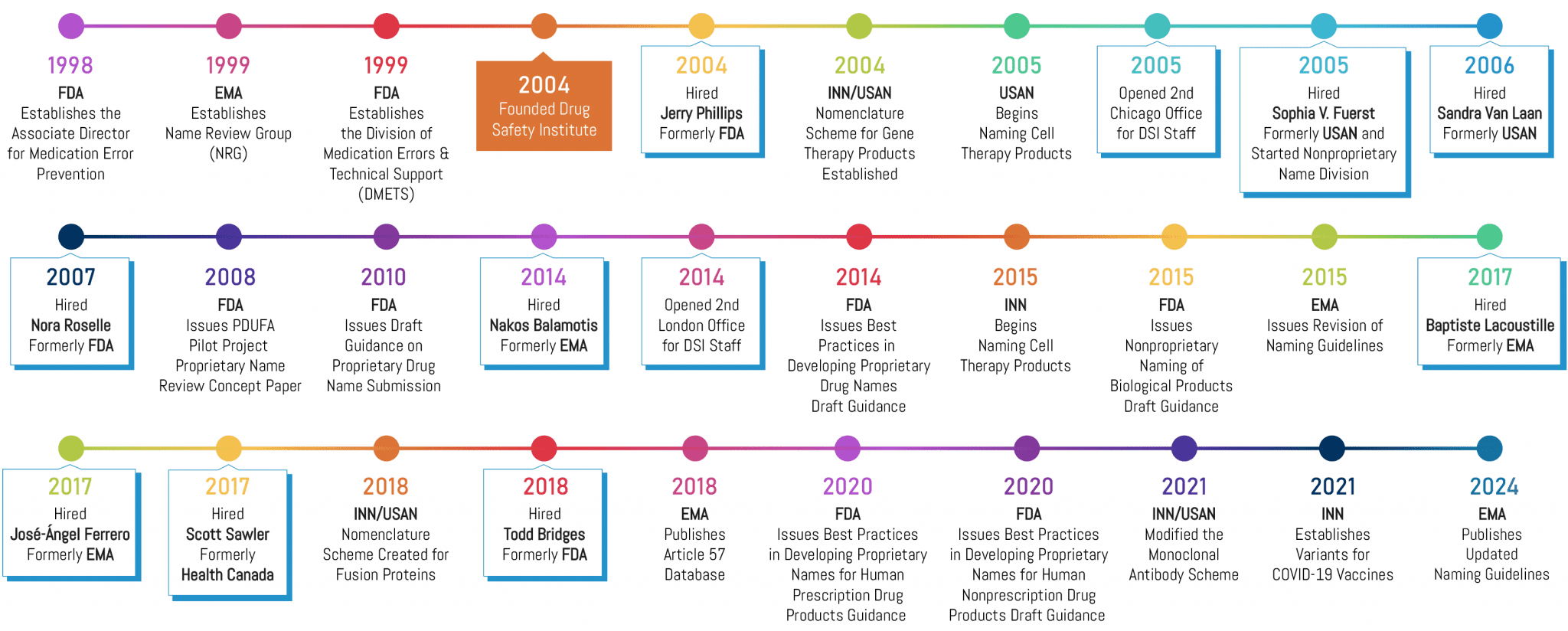
In 1999, the FDA formed the Division of Medication Errors and Technical Support (DMETS), signaling that the agency was laser-focused on reducing naming-related medication errors. At that time, Brand Institute started developing a name safety research methodology that would be fully compliant with the agency’s guidance and expectations. In 2004, we formed Drug Safety Institute and recruited Jerry Phillips, former Acting Director of FDA’s DMETS, to lead our new subsidiary. We have since staffed DSI with a “who’s who” of regulatory experts from the Food and Drug Administration (FDA), European Medicines Agency (EMA), Health Canada (HC), American Medical Association (AMA), and the World Health Organization (WHO). These experts provide our clients with industry-leading guidance pertaining to drug name safety (i.e., preventing medication errors), packaging, and labeling. Today, we proudly partner on over 75% of new pharmaceutical brand and nonproprietary name approvals worldwide annually.

87%
FDA

76%
EMA

87%
Health Canada

76%
Japan

78%
South Korea

78%
Brazil

78%
China

71%
India


79%
INN

76%
USAN
To provide the global pharmaceutical and biotech communities with regulatory expertise, strategy, and best practices in brand name and nonproprietary (USAN/INN) name development, name safety research, and comprehensive regulatory solutions.


Global President


President, U.S. Regulatory Affairs


President, EU Regulatory Affairs


President, Canadian Regulatory Affairs


President, Nonproprietary Division


President, Regulatory Affairs, Nonproprietary


Vice President, EU Regulatory Affairs & Safety Research